Huntington's Disease Gene Therapy
Huntington's disease gene therapy. Two patients have entered the trial one who has received an injection of the therapy into the brain and one who has undergone an imitation procedure. NDs caused by polyglutamine polyQ expansion represent an. A leader in gene therapy Ohio State also has multiple studies for Parkinsons Huntingtons and aromatic L-amino acid decarboxylase deficiency a rare genetic disorder affecting children and resulting in developmental delay weak muscle tone and difficulty moving Mohler said.
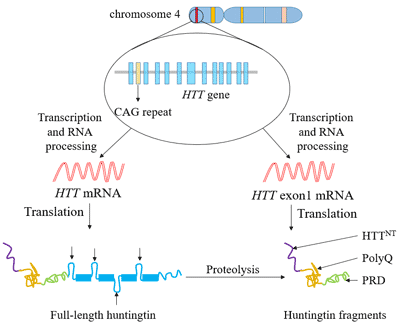
The new gene therapy that sufferers cannot afford Efforts to treat Huntingtons disease involve costly drugs way beyond the reach of the poor communities in. Muellers gene therapy strategy for Huntingtons disease. AMT-130 targets the accumulation of the exon 1.
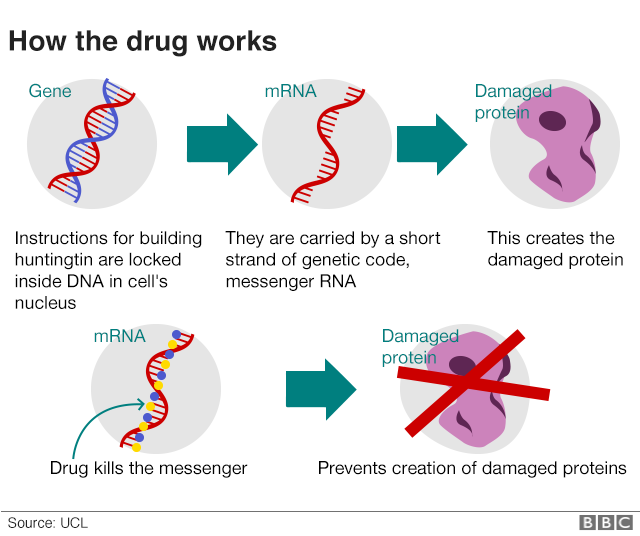
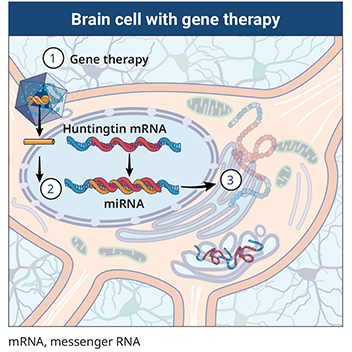
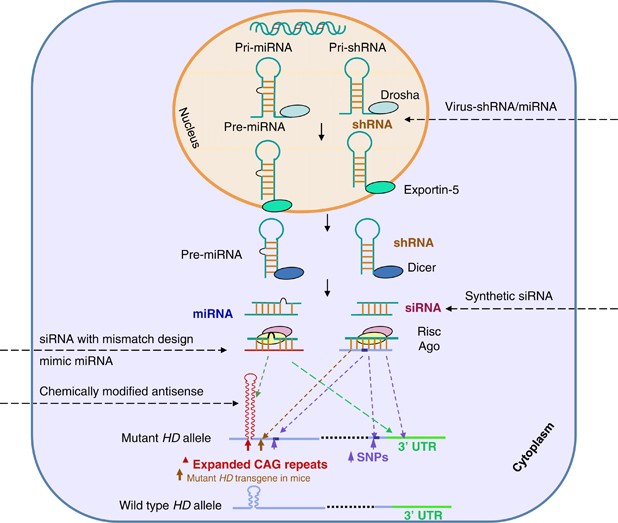
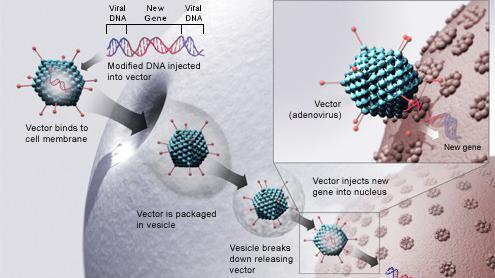
AMT-130 targets the deep brain structures known for the disease pathology onset. Using gene therapy to switch off genes instead of adding new ones could slow down or prevent the fatal brain disorder Huntingtons disease. An artificial miRNA targeting the huntingtin.
UniQures gene therapy candidate for Huntingtons disease is differentiated in that. The only approved drug is tetrabenzene which palliates motor abnormalities. AMT-130 is a gene therapy designed to lower the production of the mutated form of the huntingtin protein the underlying cause of Huntingtons disease.
Warby Alexandre Montpetit Anna R. Gene Therapy 101 Huntingtons Disease Society of America Gene Therapy 101 In addition to ongoing clinical trials of RocheGenentech and Wave huntingtin-lowering therapies a number of other companies are developing drugs aimed at lowering levels of mutant huntingtin in the body and brain. Frederic Sadou ISMScience Photo.
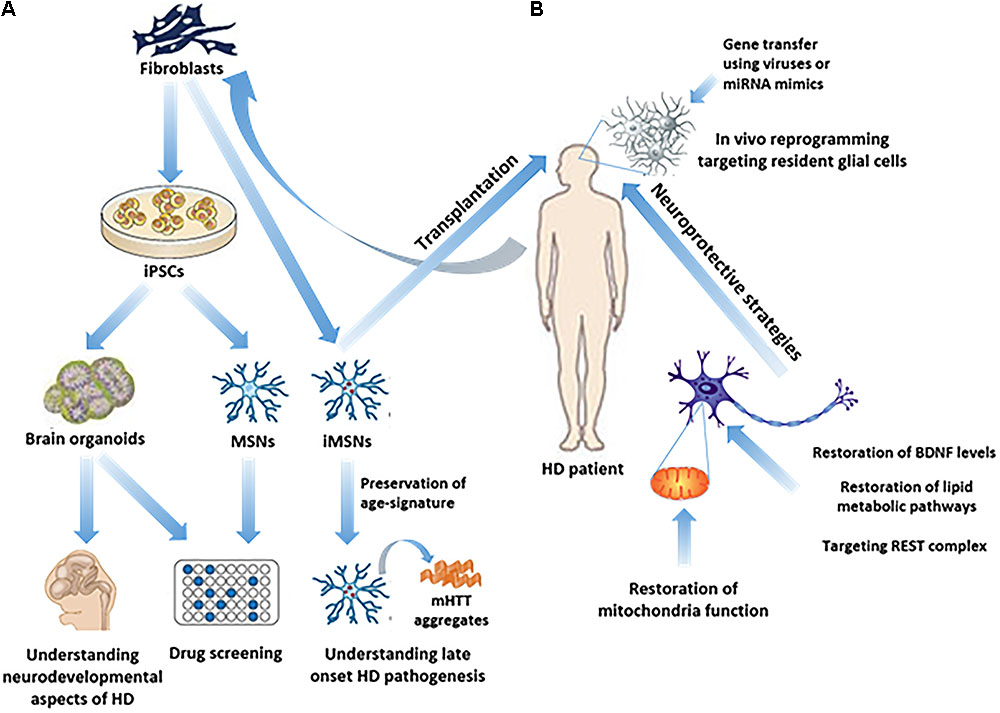
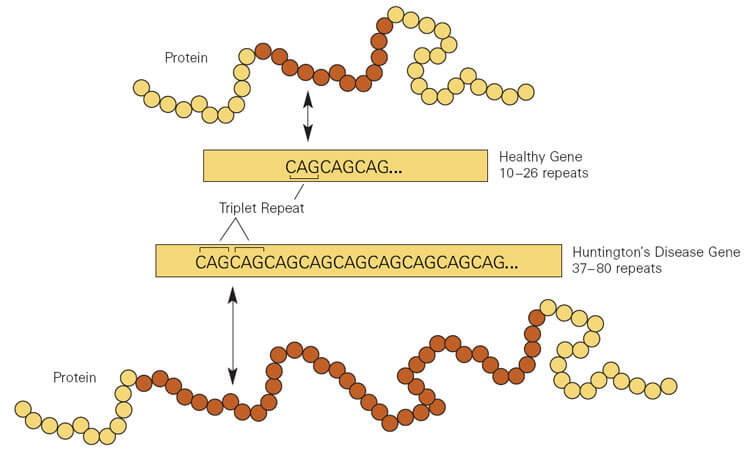
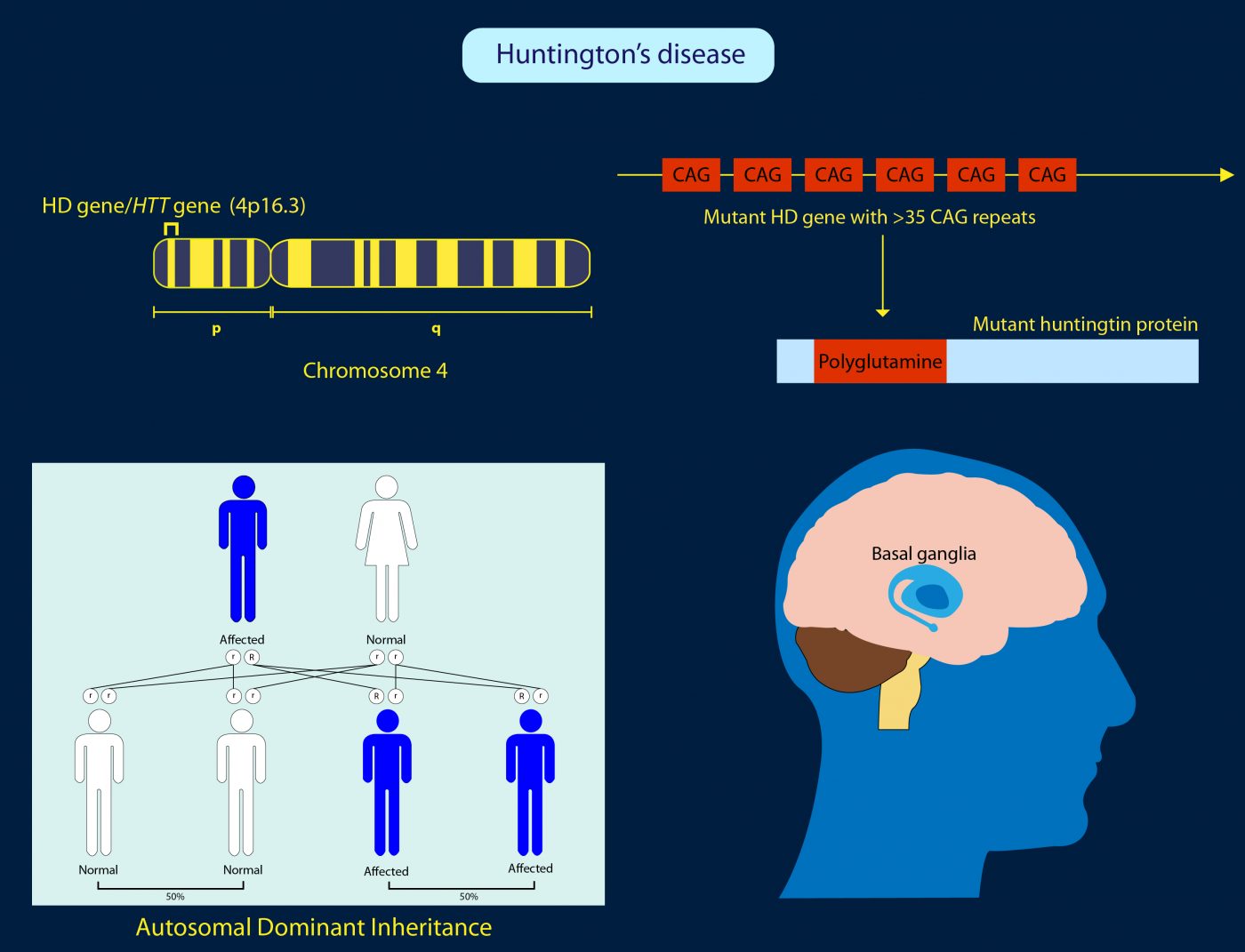
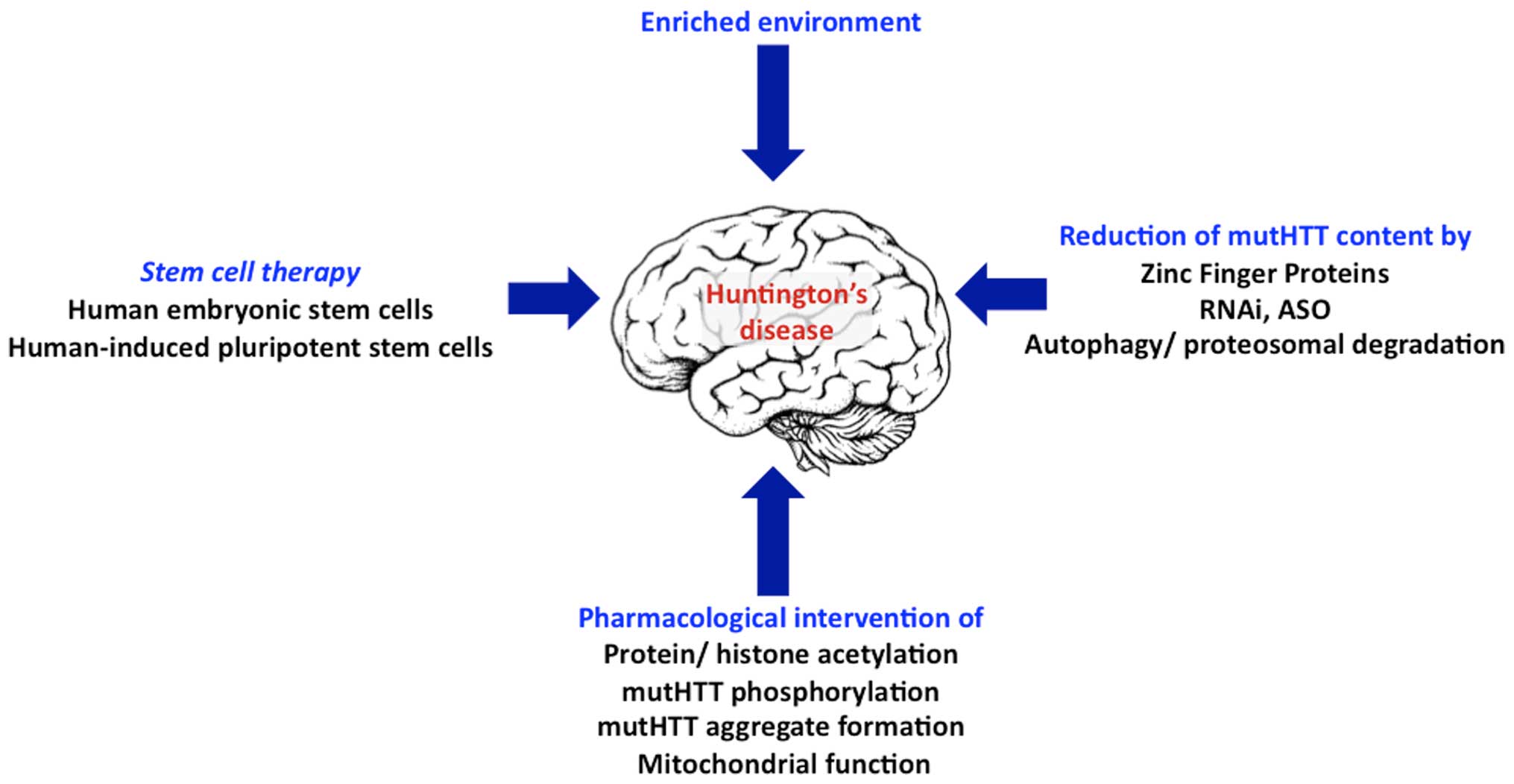
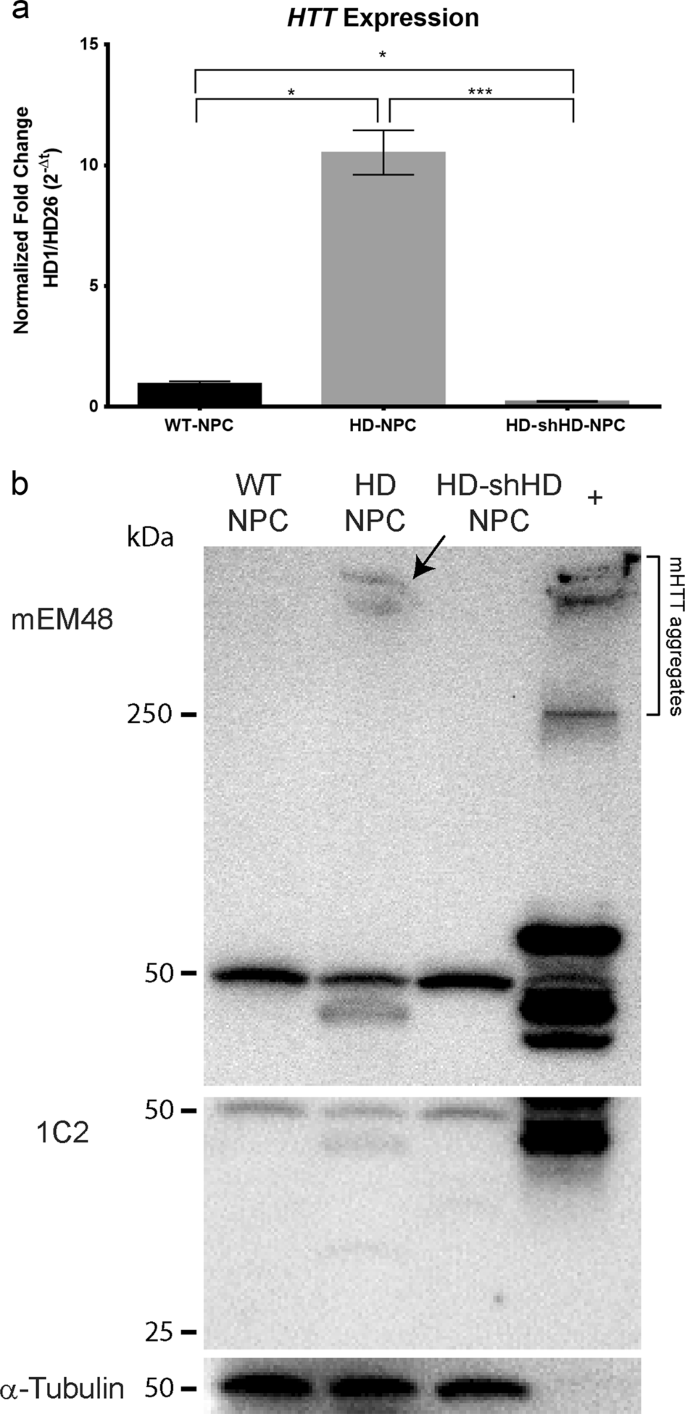
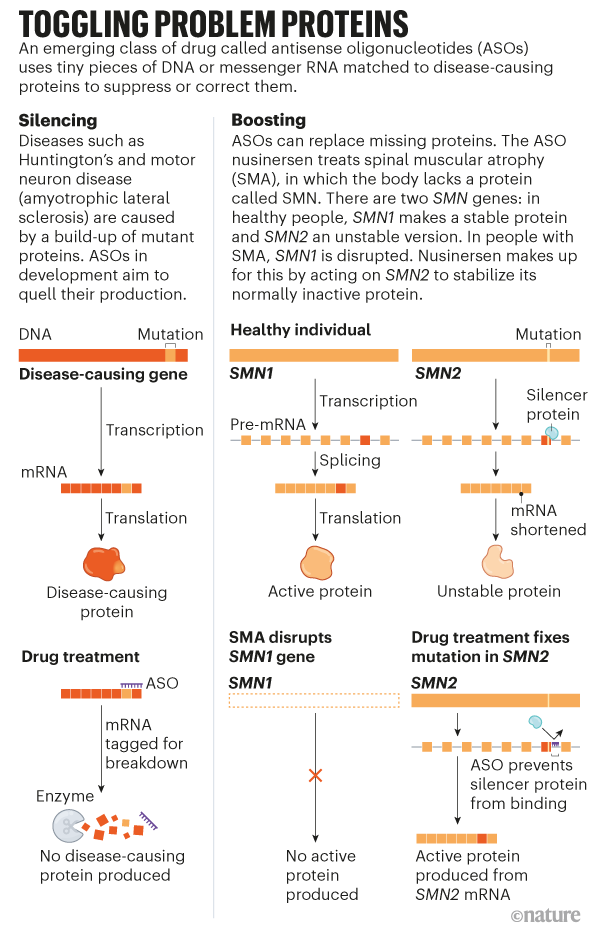
Many HD therapeutics target downstream consequences and symptoms of the causal pathogenic mutation in the Huntingtin HTT gene while a few next-generation therapies target mHTT itself see Table 1 11. Butland Henk Visscher Jennifer A. Researchers have taken an important first step toward protecting against Huntingtons disease using gene therapy.
Collins Alicia Semaka Thomas J. The method which exploits a.
Warby Alexandre Montpetit Anna R.
The only approved drug is tetrabenzene which palliates motor abnormalities. NDs caused by polyglutamine polyQ expansion represent an. Frederic Sadou ISMScience Photo. Therapies that are efficacious in animal models have to date shown benefit for humans. Huntingtons Disease is an. Gene therapy has recently undergone a renaissance with renewed expectation that transfer of genetic. The new gene therapy that sufferers cannot afford Efforts to treat Huntingtons disease involve costly drugs way beyond the reach of the poor communities in. AMT-130 targets the accumulation of the exon 1. Warby Alexandre Montpetit Anna R.
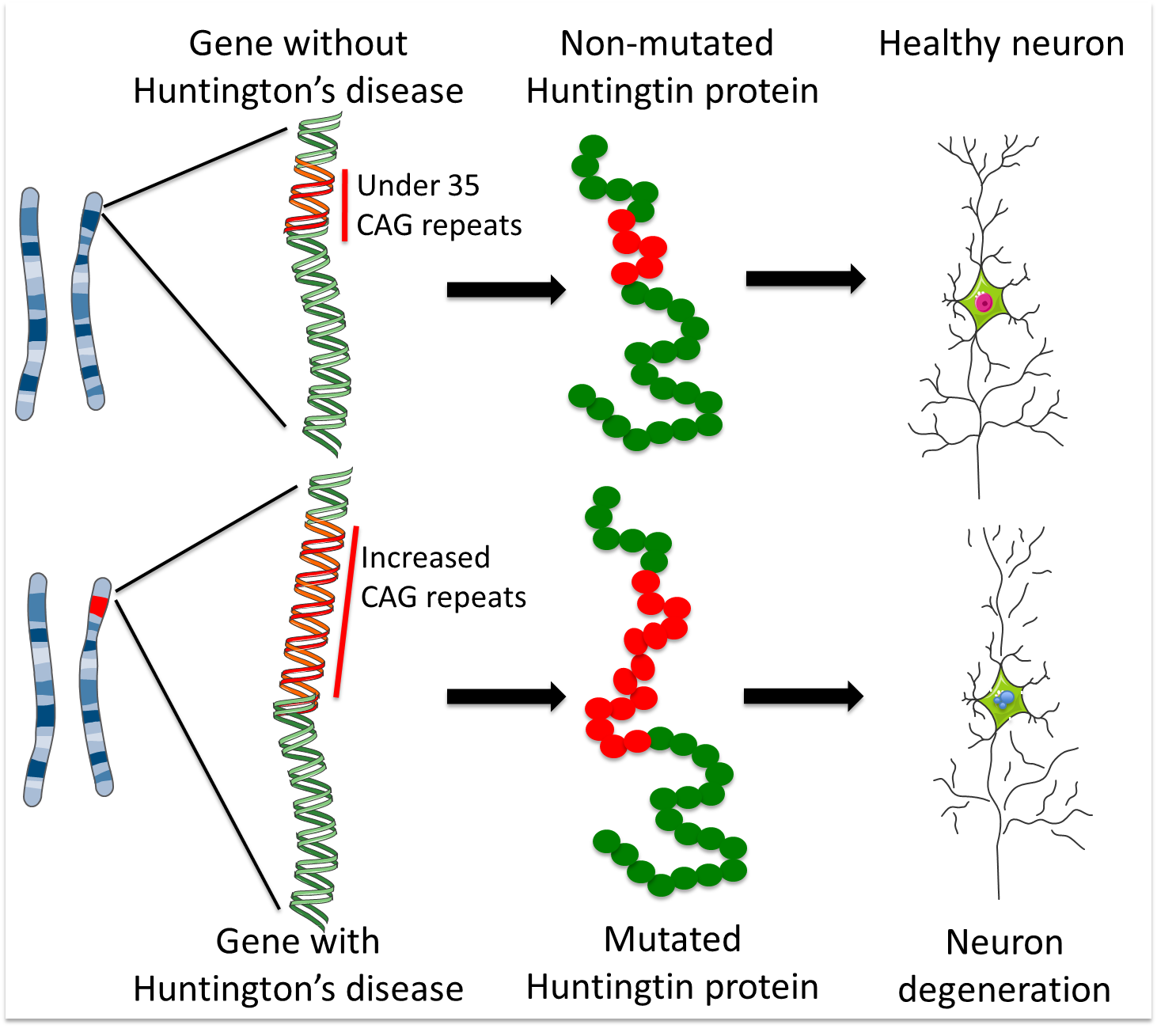
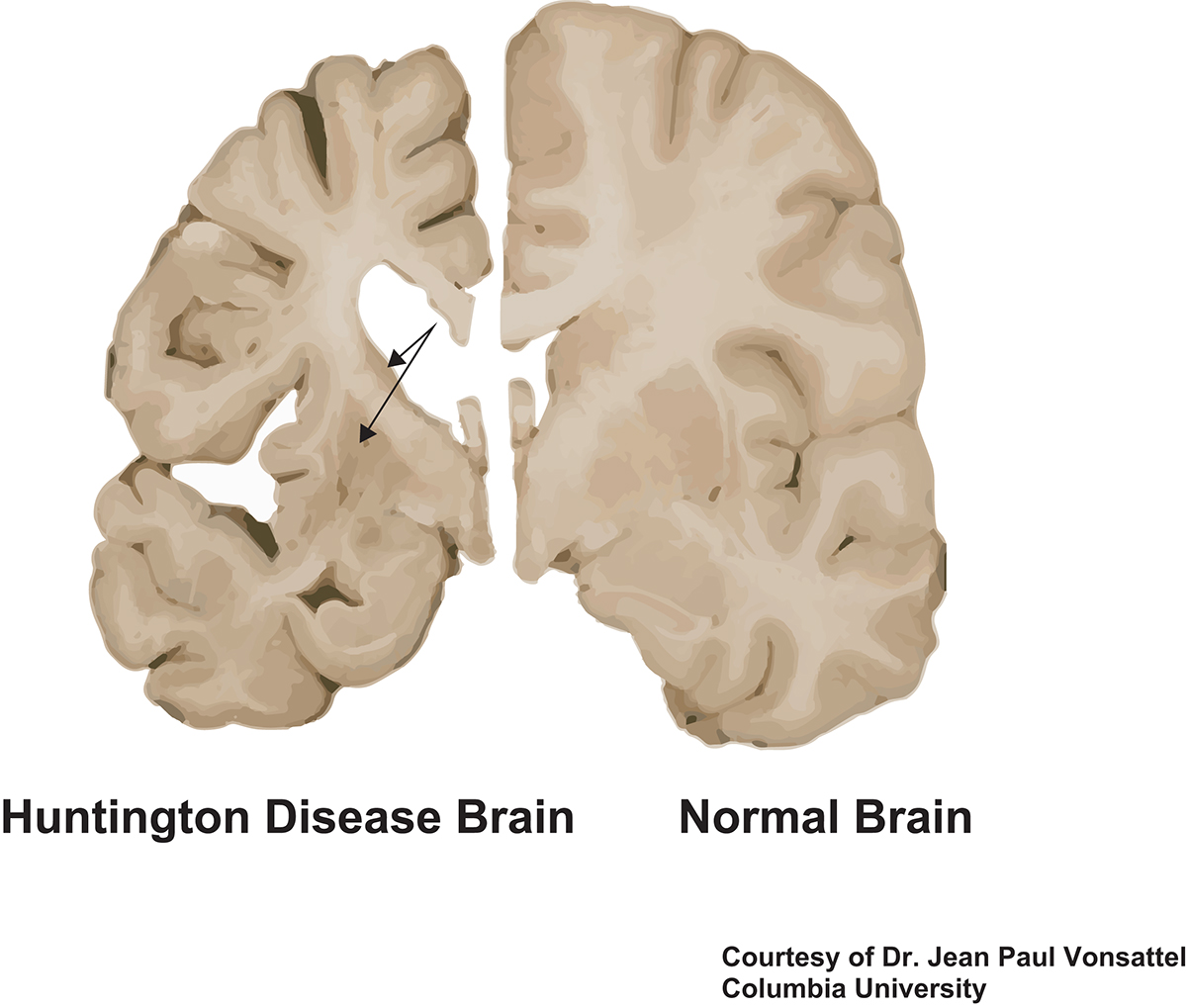
Collins Alicia Semaka Thomas J. NDs caused by polyglutamine polyQ expansion represent an. Huntingtons disease HD is a neurodegenerative disease for which there is no cure. Collins Alicia Semaka Thomas J. Gene therapy has recently undergone a renaissance with renewed expectation that transfer of genetic. AMT-130 is a gene therapy designed to lower the production of the mutated form of the huntingtin protein the underlying cause of Huntingtons disease. One possible mechanism is a diminished nuclear translocation of the transcription factor sterol regulatory element binding protein 2 SREBP2 and consequently reduced activation of SREBP-controlled genes in the cholesterol biosynthesis pathway.






















Post a Comment for "Huntington's Disease Gene Therapy"